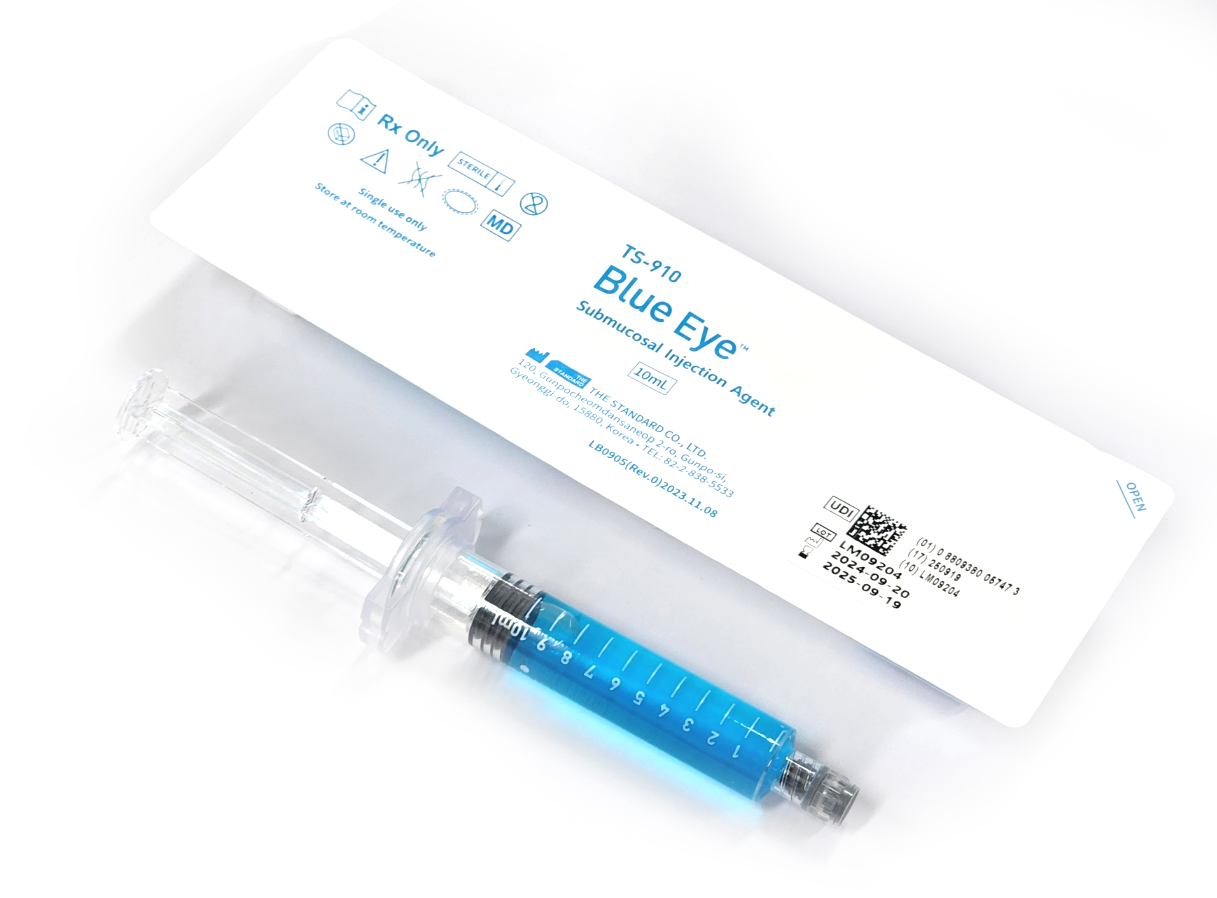
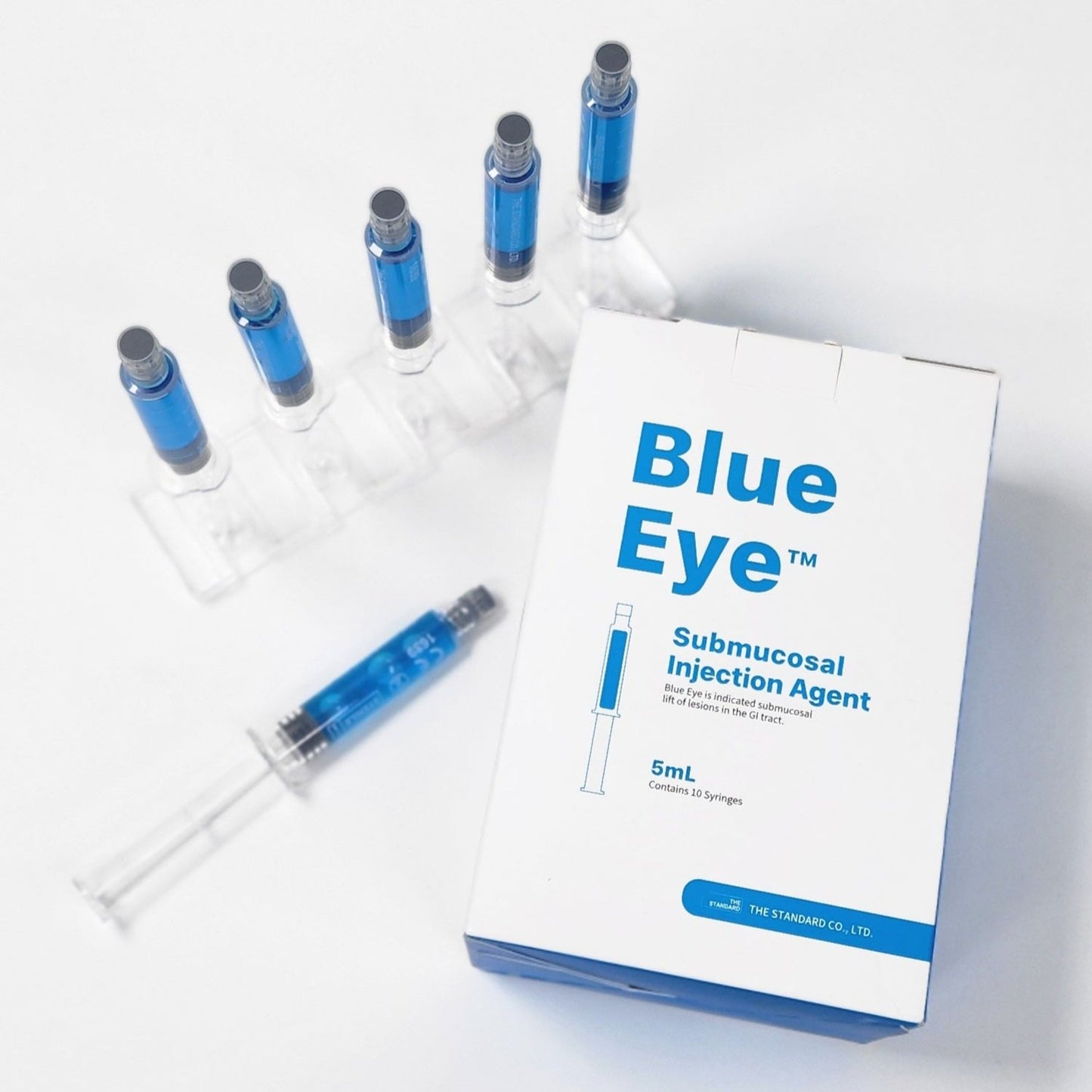
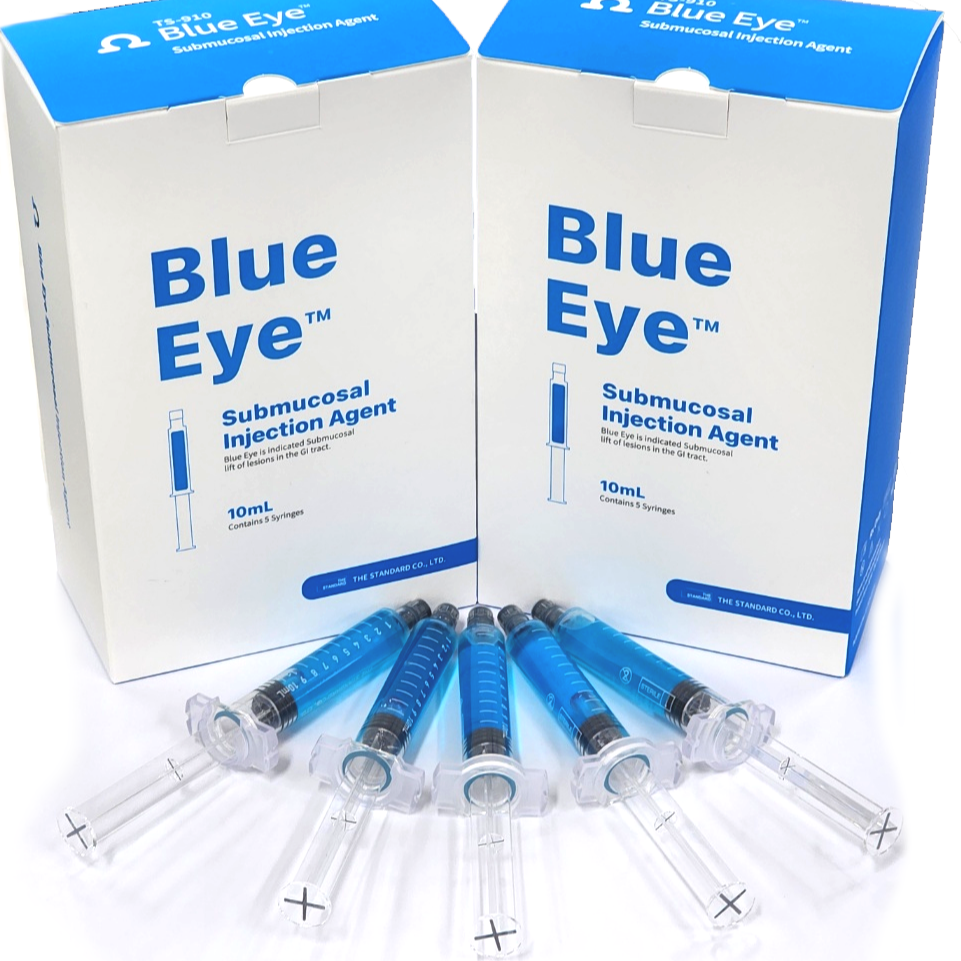
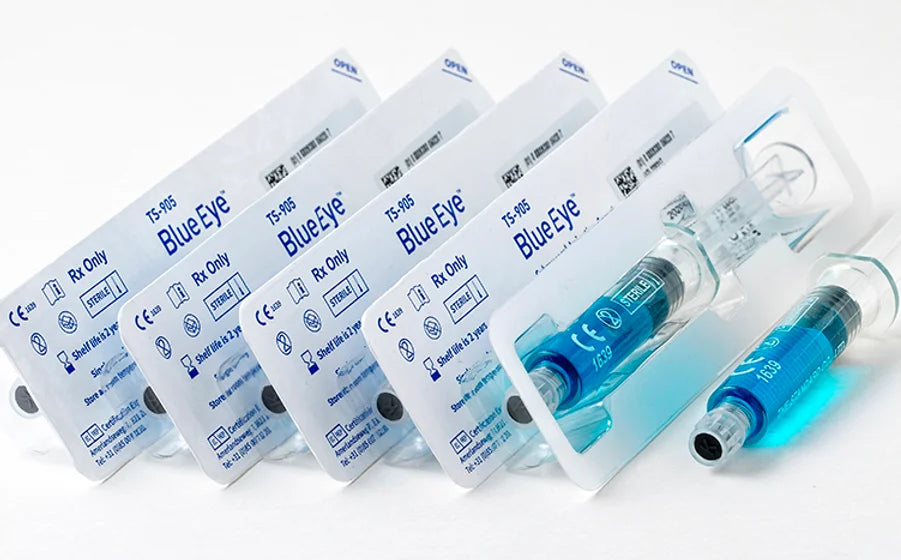
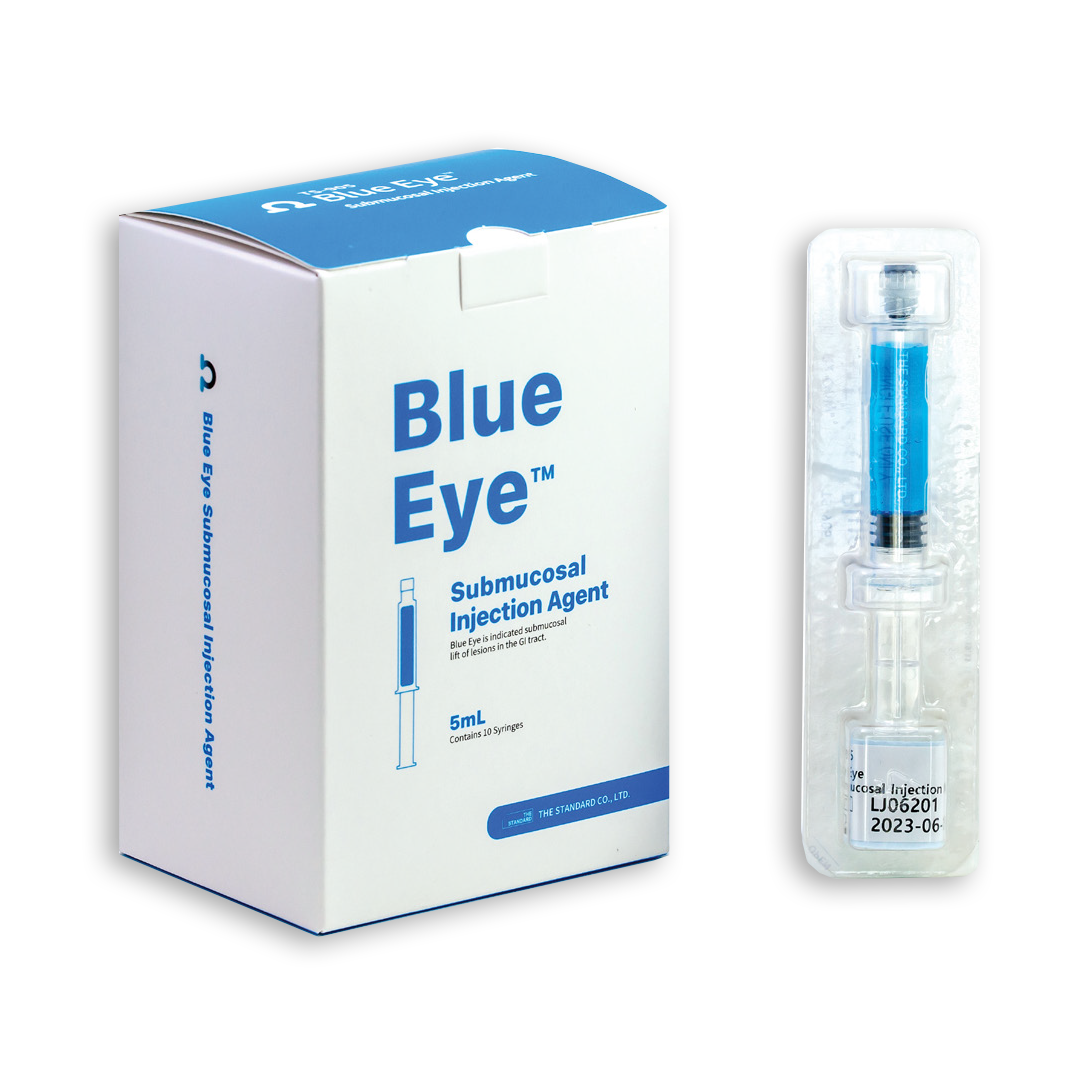
Blue Eye™ Submucosal Injection Agent
Blue Eye™ Submucosal Injection Agent is a Class II medical device, an injectable solution intended to assist in the endoscopic resection of polyps, adenomas, and other gastrointestinal lesions when needed.
SMD Wynne
Blue Eye™ Submucosal Injection Agent
Blue Eye™ Submucosal Injection Agent
Information
Information
Blue Eye™ Submucosal Injection Agent is a Class II medical device, an injectable solution intended to assist in the endoscopic resection of polyps, adenomas, and other gastrointestinal lesions when needed.
Blue Eye™ provides a helpful lift of the lesion that lasts longer for the endoscopist, and also provides improved visualization of the lesion and underlying tissue.
Blue Eye™ is expected to result in fewer injections, shortened procedure time and better outcomes.
Used since 2019, Blue Eye™ was cleared by FDA in late 2022, and became available for use by U.S. endoscopists in 2023. Some early comments from our physician customers who have used Blue Eye™ are “helpful”, “successful”, “fantastic”, and “less expensive”.
Literature
Literature
Share

-
SMD Wynne Corporation is an Ohio based importer and distributor of specialized medical devices. We have been proudly serving the healthcare industry since 1996.
-
For more information or assistance regarding Blue Eye™ or Black Eye please Call: 800-324-6090 or Email: sales@smdwynne.com





